Why do Hard-Boiled Egg Yolks Turn Green?

Hard-cooked eggs can have a green shade from overcooking. It’s harmless but doesn’t look tasty and is easy to avoid. We answer the most common questions and take a deep dive into the complete chemical process that turns an egg yolk green.
- Why do hard-boiled egg yolks turn green?
- Is it safe to eat a hard-boiled egg with a green yolk?
- What’s the complete chemical process that turns an egg yolk green when overcooking?
- What factors increase the risk of the egg yolk turning green?
- Summary
Why do hard-boiled egg yolks turn green?
The green shade on hard-boiled egg yolks is caused by overcooking. Heat makes iron in the yolk react with sulfur from the egg white. The result is dark iron sulfide, which looks greenish in combination with the bright yellow yolk. It’s safe to eat and can be avoided with slow cooking.
Is it safe to eat a hard-boiled egg with a green yolk?
Yes, it’s safe to eat a hard-cooked egg with a green-grey shade in the yolk. The green discoloration results from a reaction between the iron in the yolk and sulfur in the egg whites. Although ferrous sulfide is slightly toxic, it’s such a small amount that it’s considered safe to eat.
While harmless to eat, it might not be the tastiest egg you ever had when it’s all dark from overcooking.

How can I avoid an egg from turning green when cooking?
To avoid the egg yolk from turning green when boiling:
- keep the water at boiling temperatures or just below boiling temperatures to prevent overheating
- use a large pan and keep the eggs in a single layer
- turn off the heat when the water reaches boiling temperatures
- don’t let the eggs in the water for too long; 10-12 minutes is enough for medium size eggs
- chill the eggs with cold water immediately after cooking to stop any chemical reactions turning the yolk green
The key is to add just enough heat to make the egg hard, but not so much that it turns green.
What’s the complete chemical process that turns an egg yolk green when overcooking?
A couple of interesting biochemical processes occur before the iron can react with the sulfur to turn the egg yolk green.
Let’s go over them step by step.
Iron in the Egg Yolk
A chicken egg yolk contains 2.7% of iron, a vital nutrient for the embryo. 95% of the iron is bound to phosvitin, a protein in the egg yolk.
When the embryo starts to grow, blood vessels grow into the yolk to fetch nutrients.
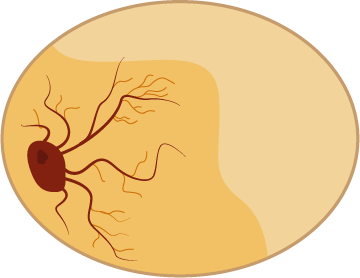
The blood contains red blood cells that use iron to carry oxygen to the developing chick.
The unborn chick is actually breathing oxygen inside the egg. The oxygen is coming through tiny pores in the eggshell. A standard chicken egg has more than 7000 pores for oxygen to pass through.
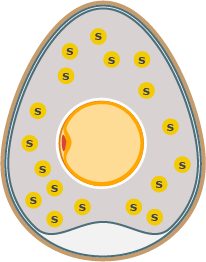
Sulfur in the Egg White
We all know sulfur as it’s the sole responsible for the pungent odor of rotten eggs.
The egg white sits around the yolk as a protective layer that kills incoming bacteria. It’s filled with water and proteins. More than half of the egg white consists of the protein ovalbumin, a protein that contains free sulfhydryl groups containing sulfur.
Cysteine
Egg proteins are long chains of amino acids. Most of the sulfur in chicken eggs is contained in the essential amino acid methionine, a precursor of the amino acid cysteine.
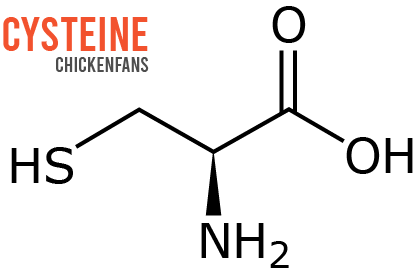
In humans, cysteine plays an essential role in the digestion of alcohol. It became popular in 2020 when scientists discovered cysteine could alleviate alcohol-related hangover symptoms, such as nausea and headaches. The sulfur-containing cysteine in eggs cures hangovers.
Heating the Egg
When the egg is cold, the vitelline membrane is a barrier that keeps the chemicals in the yolk separate from the egg white. But when you start to cook the egg, a couple of magical things happen.
First of all, the heat makes the proteins in the raw egg unfold and form new bonds with each other. This process is called denaturation and is the reason why the egg becomes hard when you boil it.
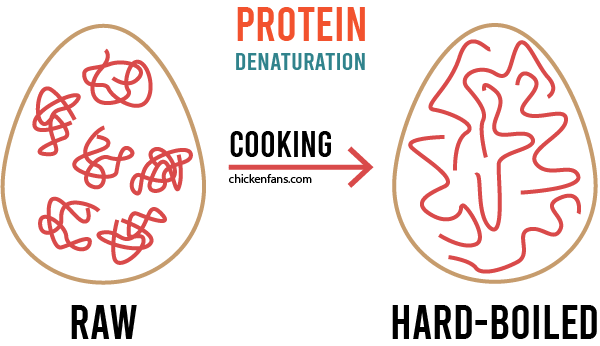
Because of all the untangling, sulfur is released from the amino acids. It starts to form hydrogen sulfide, a gas that smells like rotten eggs. We’re lucky it’s such a small amount of gas, or we would not be eating eggs, ever.
We all know what happens with a soda if we leave it in the sun for too long: the gas escapes. The same happens with hydrogen sulfide, it tries to escape from the egg-white. There are not too many places for the gas to go, so it tries to diffuse into the egg yolk.
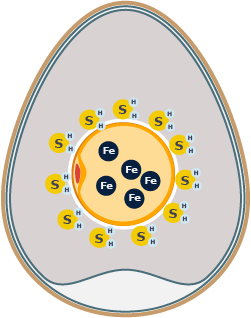
When you heat the egg long enough and at high temperatures, the otherwise strong phosvitin proteins in the yolk start to break down through hydrolysis. The phosvitin can’t hold on to the iron, and the iron is released into the yolk.
Iron reacting with Sulfur
The iron (Fe) from the yolk meets the sulfur (S) from the egg white on the yolk’s edge, where the vitelline membrane is falling apart. The chemical reaction produces ferrous sulfide (FeS).
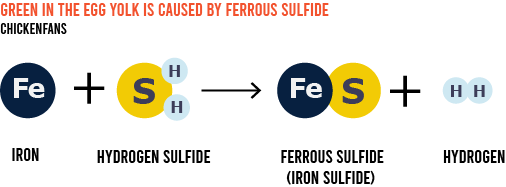
Ferrous sulfide is a dark-colored iron sulfide that looks green when mixed with the yellow yolk. The final result is the greenish-black discoloration you get in the hard-cooked egg.
Some sources claim the green is ferric sulfide, but that’s an unstable artificial material that does not occur in nature and decays in ferrous sulfide.
What factors increase the risk of the egg yolk turning green?
The risk for a grey-green discoloration of the egg yolk increases when:
- the egg is cooked at very high temperatures
- the egg is heated for a long time
- the egg is stored long before cooking
- the egg yolk has high pH-levels
- you cook the eggs in an iron pan
The pH levels of the egg increase when the egg gets older. The pH can shift to alkaline values, with carbon dioxide leaving the egg in a few days. This increases the risk that the yolk’s iron reacts with the egg white’s sulfur.
Since iron is turning the egg green, it’s better to avoid cooking them in a cast iron skillet.
The chicken breed, egg size, egg color, and egg quality do not affect the green discoloration of the yolk.

Summary
The grey-green discoloration of the egg yolk in hard-boiled eggs is caused by overcooking. The heat makes the iron in the egg yolks react with the sulfur in the egg whites. The resulting dark ferrous sulfide looks green on top of the yellow egg yolk.
To avoid the green tinge, it’s key to prevent the iron in the yolk from being released. Lower the water temperature and ensure the egg is only heated long enough to make it hard. Immediately chill it with cold water after cooking.





















